|
[home] [subscription form] [cover story] [introduction] [people and places] [medical spa destinations]
|
||
The AUSTIN-WESTON CENTER for cosmetic surgery
is essentialy a team of talented surgeons instead of just one
 Cosmetic Surgeons Contribute to Silicone Breast Implant Ruling
Cosmetic Surgeons Contribute to Silicone Breast Implant Ruling
New data leads to the FDA decision to lift fourteen year ban
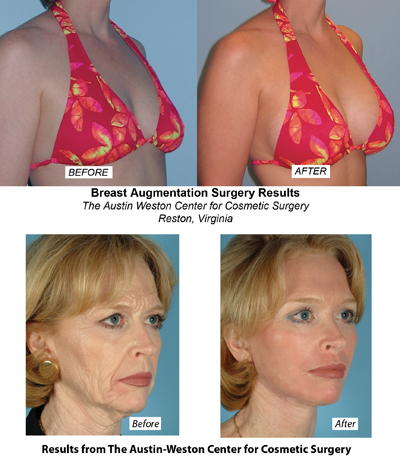
 Despite the ban on silicone implants in effect since 1992, some cosmetic surgery centers in the U.S. have continued to use them in participation with the FDA for their research studies on the safety of the controversial material. The Austin-Weston Center for Cosmetic Surgery of Reston, VA has been a part of an adjunct study for the past ten years with Mentor, one of the two implant manufacturers approved by the FDA for participation. Mentor and Allergan were both asked to gather information for the FDA and the results of their core studies led to the decision on November 17, 2006 to restore silicone implants to the market.
Despite the ban on silicone implants in effect since 1992, some cosmetic surgery centers in the U.S. have continued to use them in participation with the FDA for their research studies on the safety of the controversial material. The Austin-Weston Center for Cosmetic Surgery of Reston, VA has been a part of an adjunct study for the past ten years with Mentor, one of the two implant manufacturers approved by the FDA for participation. Mentor and Allergan were both asked to gather information for the FDA and the results of their core studies led to the decision on November 17, 2006 to restore silicone implants to the market.
Dr. Robert Sigal, one of the three partners in The Austin-Weston Center has been a part of the study since 1996. “In the early 90’s there were concerns that silicone implants were linked to a series of diseases. Early data was anecdotal and there was not a lot of good information available,” notes Sigal. “There were questions about rupture rates, re-operation rates and the possible link to diseases such as fibromyalgia and arthritis. Epidemiologic studies were conducted on whether diseases were occurring at a rate more often than those found in women without the implants.”
Approximately five percent of breast augmentation patients at The Austin-Weston Center have been participants in the silicone study under manufacturer Mentor. Certain criteria had to be met by women to participate: they had to be over age 22; they had to have either failed an attempt with saline implants, due to tissue thinness or rippling of the implant; or they had drooping in the breast that needed lifting only achievable with silicone implants. The patients were then followed over time to see if they developed any diseases at a rate different from saline implant recipients or the general population.
Despite complications that can arise from any implants including rupture, breast pain and tissue hardening, increases in rates of cancer or connective tissue disease were not found associated with silicone. “The data is not conclusive, but strong enough to bring implants back on the market,” says Sigal. “The bottom line for the FDA was that if patients were appropriately informed that the implants aren’t lifetime devices and they may need additional surgery, this was satisfactory.” Mentor and Allergan will continue to follow 32,000 patients for 10 years.
After a 90 day phase-in period, the silicone implants will be widely available once again. Will women be willing to take the plunge back into silicone? "Some people are still going to be against silicone even after the approval; others will feel confident in the ruling,” says Sigal. “Our role as doctors is to give information and put it into context.” Now that silicone is back on the market, anatomy, tissue thickness and the patient’s preference all will play a role in choosing the implant type. The benefits of silicone implants are a more natural look and feel due to the make-up of the gel material.
“The important thing is that the medical community was able to take a step back from this highly-charged issue, take the time to collect data and come to a reasonable conclusion based in science and statistics. More options are available to women now, and that’s always a good thing,” concludes Sigal.
|
|
|||